Mummified baboons reveal the far reach of early Egyptian mariners
Abstract
The Red Sea was witness to important events during human history, including the first long steps in a trade network (the spice route) that would drive maritime technology and shape geopolitical fortunes for thousands of years. Punt was a pivotal early node in the rise of this enterprise, serving as an important emporium for luxury goods, including sacred baboons (Papio hamadryas), but its location is disputed. Here, we use geospatial variation in the oxygen and strontium isotope ratios of 155 baboons from 77 locations to estimate the geoprovenance of mummified baboons recovered from ancient Egyptian temples and tombs. Five Ptolemaic specimens of P. anubis (404–40 BC) showed evidence of long-term residency in Egypt prior to mummification, consistent with a captive breeding program. Two New Kingdom specimens of P. hamadryas were sourced to a region that encompasses much of present-day Ethiopia, Eritrea, and Djibouti, and portions of Somalia and Yemen. This result is a testament to the tremendous reach of Egyptian seafaring during the 2nd millennium BC. It also corroborates the balance of scholarly conjecture on the location of Punt.
eLife digest
Strontium is a chemical element that can act as a geographic fingerprint: its composition differs between locations, and as it enters the food chain, it can help to retrace the life history of extant or past animals. In particular, strontium in teeth – which stop to develop early – can reveal where an individual was born; strontium in bone and hair, on the other hand, can show where it lived just before death. Together, these analyses may hold the key to archaeological mysteries, such as the location of a long-lost kingdom revered by ancient Egyptians.
For hundreds of years, the Land of Punt was one of Egypt's strongest trading partners, and a place from which to import premium incense and prized monkeys. Travellers could reach Punt by venturing south and east of Egypt, suggesting that the kingdom occupied the southern Red Sea region. Yet its exact location is still highly debated.
To investigate, Dominy et al. examined the mummies of baboons present in ancient Egyptian tombs, and compared the strontium compositions of the bones, hair and teeth of these remains with the ones found in baboons living in various regions across Africa. This shed a light on the origins of the ancient baboons: while some were probably raised in captivity in Egypt, others were born in modern Ethiopia, Eritrea, Djibouti, Somalia and Yemen – areas already highlighted as potential locations for the Land of Punt.
The work by Dominy et al. helps to better understand the ancient trade routes that shaped geopolitical fortunes for millennia. It also highlights the need for further archaeological research in Eritrea and Somalia, two areas which are currently understudied.
Introduction
The sacred baboon (Papio hamadryas) was a recurring motif in ancient Egyptian art and religion, from Predynastic statuettes to later mortuary traditions, including wall paintings, reliefs, amulets, and statues—a tradition exceeding 3000 years (Figure 1). In most cases, P. hamadryas was the embodiment of Thoth, a deity associated with the moon and wisdom. It is a rare example of apotheosis among nonhuman primates. The archetype of this manifestation—a male baboon in a seated posture, hands on knees, and often surmounted with a lunar disc or crescent—was strikingly consistent for millennia. Figurines of seated baboons even spread into the Levant and across the Mediterranean during the Middle Bronze Age, but the biological realism diminished with increasing distance from Egypt, becoming symbolic renderings rather than species-specific representations (Dothan and Regev, 2011). Such a pattern invites two complementary interpretations: first, that ancient Egyptian artists were concerned with species-level realism and second, that they were themselves witness to the plants and animals in their works. Accordingly, some ecologists have viewed the artistic record of Egypt as a biological survey and used it to assess ecosystem stability through time (Yeakel et al., 2014).

Egyptian iconography of Papio hamadryas, a tradition exceeding 3000 years.
(a) Statuette inscribed with the name of King Narmer, Early Dynastic Period, 1st Dynasty, ca. 3150–3100 BC (no. ÄM 22607, reproduced with permission from the Ägyptisches Museum und Papyrussammlung, 2020, under the terms of a CC0 1.0 license. (b) Bronze axe head, Middle Kingdom, 12th or 13th Dynasty, ca. 1981–1640 BC (no. 30.8.111, reproduced with permission from The Metropolitan Museum of Art, 2020, under the terms of a CC0 1.0 license. (c) Reliefs at the mortuary temple of Queen Hatshepsut [Deir el-Bahari]. A hamadryas baboon sits in the rigging of a ship. It is one of five being imported from Punt; New Kingdom, 18th Dynasty, ca. 1473–1458 BC. (d) Wall painting in the mortuary chapel of Rekhmire (TT 100), Vizier to Tuthmose III and Amenhotep II. A baboon (P. hamadryas) is shown as tribute in a procession from Nubia. Three vervets (Chlorocebus aethiops) are also illustrated, one of which climbs the neck of a beautifully rendered giraffe; New Kingdom, 18th Dynasty, ca. 1479–1425 BC. (e) Large 35-ton statue at Hermopolis Magna (author NJD shown for scale); erected by Amenhotep III, New Kingdom, 18th Dynasty, ca. 1370 BC. (f) Frieze of baboons on the east-facing facade of the rock-cut temple of Abu Simbel (g), New Kingdom, 18th Dynasty, ca. 1265 BC. The raised arms are interpreted as a posture of adoration toward the rising sun, whereas the open mouth may represent vocal behavior (te Velde, 1988). (h) Pectoral necklace of Tutankhamun; baboons are surmounted with lunar disks and simultaneously adoring the central solar disk, a rare combination of two stereotypical postures; New Kingdom, 18th Dynasty, ca. 1341–1323 BC (no. JE 61885, Museum of Egyptian Antiquities). (i) Faience figurine and exemplary representation of Thoth: a male P. hamadryas in a seated posture, hands on knees, and surmounted by a lunar disc, Ptolemaic period, 332–30 BC (no. E 17496, Musée du Louvre).
© 2020, Rama, Creative Commons Attribution-ShareAlike 3.0. Figure 1I is reproduced with permission from Rama, 2020, under the terms of the Creative Commons Attribution-ShareAlike 3.0; this image is not distributed under the terms of the CC0 1.0 license, and further reproduction of this image panel should adhere to the terms of the CC-BY-SA 3.0 license.
© 2019, Sandro Vannini. All rights reserved. Figure 1D is reproduced with permission from Sandro Vannini, 2019; this image is not distributed under the terms of the CC0 1.0 license, and further reproduction of this image panel would need permission from the copyright holder.
Yet, the Holocene fossil record of Egypt is devoid of any monkey species, let alone P. hamadryas (Geraads, 1987). Gaps in the fossil record are common, of course, and seldom conclusive on the question of regional absence, but the premise is reinforced by ecological modeling (Chala et al., 2019), which indicates little appreciable change to the distributions of baboons during the past 20,000 years. Such evidence distinguishes P. hamadryas as the only animal member of the Egyptian pantheon that is naturally absent from Egypt today and during antiquity. Setting aside the puzzling question of why ancient Egyptians deified P. hamadryas (Thomas, 1979), the level of reverence was sufficient to justify the importation, husbandry, and mummification of it and another species, P. anubis, the olive baboon. (See Box 1 on the biogeography of Papio).
Box 1.
Biogeography of baboons.
The taxonomy of baboons is a topic of enduring debate, with major types classified as either species or allopatric subspecies of the superspecies Papio hamadryas (Jolly, 1993). The distinction is essentially a matter of philosophy, and here we follow Zinner et al., 2013 by recognizing six phenotypically distinct allotaxa as species: the sacred or hamadryas baboon (P. hamadryas), the olive baboon (P. anubis), the yellow baboon (P. cynocephalus), the chacma baboon (P. ursinus), the Kinda baboon (P. kindae), and the Guinea baboon (P. papio). Still, these allomorphs interbreed freely in areas of sympatry, and molecular studies report widespread mitochondrial paraphyly within and between the northern and southern clades, suggesting a long history of introgressive hybridization.
The distributions of P. anubis and P. hamadryas differ, a fact that bears on the trade networks that supplied living baboons to Egypt (Figure 2). Assuming little change to these distributions over the past 5000 years (Chala et al., 2019), then P. anubis was readily available via overland trade, whereas P. hamadryas was more practically obtained via maritime trade—indeed, the distinction is apparent on temple walls, insofar as P. hamadryas is the only baboon associated with long-distance seafaring (Figure 1c). The problem that motivates us lies with the contested nautical range of Egyptian ships (Wicker, 1998), and the untapped potential of baboons to reveal the geography of early maritime trade in relation to Punt, a fabled emporium. Our goal is to use isotopic mapping to determine the geoprovenance of mummified baboons recovered from New Kingdom temples and Ptolemaic catacombs; but first, a brief chronology is necessary to introduce the specimens, as the cultural context and availability of each species varied through time (Figure 2).
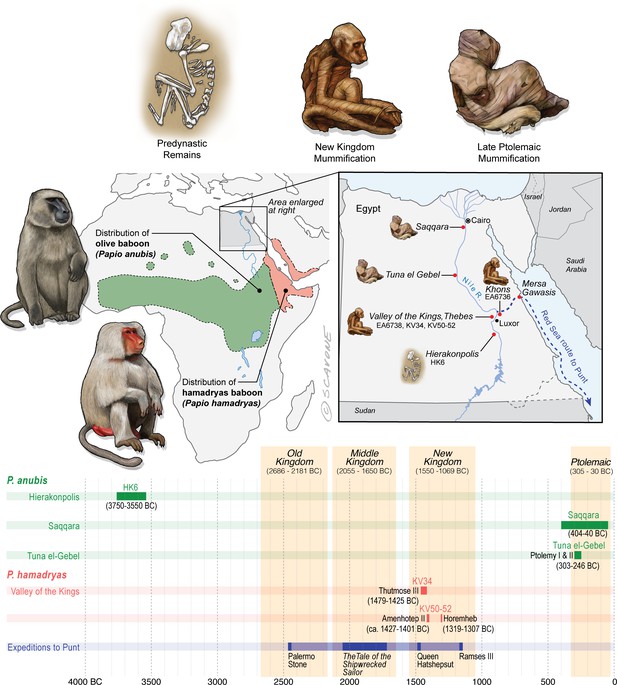
Egypt lies well beyond the distributions of P. anubis and P. hamadryas, and there is no evidence of natural populations in Egypt during antiquity.
The remains of baboons in Egypt are therefore interpreted as evidence of foreign trade. This figure puts the present study specimens—EA6736, EA6738, and those of Saqqara—into context by illustrating …
Predynastic specimens
Skeletal remains of P. anubis (n = 16) are present at the cemetery site HK6, Hierakonpolis (3750–3550 BC) (Van Neer et al., 2017). The assemblage contains adults and juveniles of both sexes, buried singly and in groups. A juvenile baboon (age: 4–5 years) was interred with an adolescent person (age: 10–15 years), suggesting status as a pet (Van Neer et al., 2004). The prevalence of skeletal pathologies points to physical abuse under captive conditions (Van Neer et al., 2017). The absence of P. anubis at every other Egyptian site of this time period, both archaeological and non-archaeological, together with the presence of two elephants at HK6, is interpreted as evidence of overland animal trade with peoples farther south in present-day Sudan (Van Neer et al., 2004).
New Kingdom specimens
In 1837, the British Museum purchased two mummified baboons from the estate of Henry Salt, British Consul-General in Egypt from 1816 to 1827. The given provenance of each specimen—Temple of Khons (EA6736; Figure 3a) and Thebes (EA6738; Figure 3c)—is itself sufficient to impute a (late) New Kingdom origin, an inference bolstered by two lines of typological evidence illustrated in Figure 2. First, the species attribution is P. hamadryas (Anderson and de Winton, 1902), the subject of numerous New Kingdom wall paintings and reliefs, some of which depict the act of importation from distant polities (Appendix 1—figure 1). Second, the seated posture and right-curled tail of EA6736 resembles the state of five mummified P. hamadryas found in the Valley of the Kings in 1906 (Davis et al., 1908; Lortet and Gaillard, 1909). The baboon-bearing tombs (KV50, KV51, KV52) are attributed on the basis of proximity to Amenhotep II (ca. 1427–1401 BC) or King Horemheb (1319–1307 BC), both members of the 18th Dynasty (Ikram, 2005). A third line of evidence concerns ante-mortem canine extraction (Figure 3), an idiosyncrasy that unites EA6736 and EA6738 with other specimens from the New Kingdom period. (See Box 2 and Appendix 1—figure 2 on the New Kingdom practice of canine extraction).

The British Museum holds two mummified baboons with New Kingdom attributions.
(a) EA6736 is attributed to P. hamadryas (Anderson and de Winton, 1902). The present analysis is based on six strands of hair sourced from the upper right arm. (b) EA6736 was the subject of an early …
Box 2.
Canine teeth and their extraction.
The extraction of canines in vivo was a prudent safety precaution—a single bite from an adult male baboon can cut human thigh muscle to the bone (Walker, 1984), and the available evidence points to regular interactions between humans and baboons at close quarters. Scores of New Kingdom paintings depict P. hamadryas working with people, often in a utilitarian role (as police animals; as fruit-harvesters [d'Abbadie, 1964; d'Abbadie, 1965; d'Abbadie, 1966; Houlihan, 1997; Osborn and Osbornová, 1998; Deputte and Anderson, 2009]), whereas some mummified individuals are interpreted as royal pets (Ikram, 2004) due to the high quality of mummification (Lortet and Gaillard, 1909) and close association with royal tombs (Davis et al., 1908). Canine extraction is evident in specimens of P. hamadryas from three royal tombs—KV34 (Thutmose III), KV51, and KV52—in the Valley of the Kings. It is also evident in the assemblage of baboons from nearby Gabanet el Giroud (Lortet and Gaillard, 1907), containing P. hamadryas (n = 4 females, two males), P. anubis (n = 5 females, one male), and five indeterminate juveniles. It has some New Kingdom affinities (Lortet and Gaillard, 1907), but radiocarbon dating of one specimen (MHNL 90001206; Musée des Confluences, Lyon) produced a date range of 803–544 BC (Porcier et al., 2019). The assemblage features numerous osteopathologies and examples of burial in lieu of formal mummification. In general, scholars tend to view canine extraction as a measure of human esteem because it speaks to close human-baboon interactions.
Late and Ptolemaic specimens
Excavations in 1968 revealed a catacomb in the sacred animal necropolis of North Saqqara (Figure 2 and see Appendix 1—figure 3), containing the remains of olive baboons (P. anubis; n = 143), vervets (Chlorocebus aethiops; n = 2), and Barbary macaques (Macaca sylvanus; n = 21) (Goudsmit and Brandon-Jones, 1999). Inscriptions indicate use from ca. 404 to 40 BC (Davies, 2006). The sample of P. anubis includes every age class, a demographic pattern consistent with captive breeding; however, the 2:1 ratio of males to females indicates sex-biased internment (Goudsmit and Brandon-Jones, 1999). An outstanding feature of the site is the preservation of six obituaries written in demotic script. In one example, an animal named Harnufi (and later, Djeho-the-baboon, a sacred by-name) was imported into Egypt in year 6 of Ptolemy V (200/199 BC) and buried on 06 September 168 BC (Ray, 2011), a minimum captive span of 31 years. In other examples, we learn that animals were brought from Alexandria or the temple estate of Ptah-under-his-moringa-tree in Memphis, where they were born, housed, and mummified (Ray, 2011). Most of the baboons (82%) have craniodental or developmental anomalies associated with vitamin D deficiency and prolonged indoor confinement (Goudsmit and Brandon-Jones, 1999).
Mummified baboons are also known from the subterranean galleries of Tuna el-Gebel (Figure 2 and see Appendix 1—figure 3), dating from the reigns of Ptolemy I and II (303–246 BC) (von den Driesch, 1993b). The assemblage was described in 1989 (82 specimens of P. anubis, Kessler, 1989) and again in 2005 (>200 specimens, including one P. hamadryas [Kessler and Nur el-Din, 2005]). The total number of burials is estimated at ∼2000 (Kessler and Nur el-Din, 2005). The initial data set contained a comparable number of adult males (n = 34) and females (n = 28), which hints at captive breeding, yet it was devoid of animals < 2 years of age. Severe bone deformations and chronic degenerative pathologies in 38 individuals suggest extended captivity under abject conditions (von den Driesch and Boessneck, 1985; von den Driesch, 1993a; Nerlich et al., 1993).
This treatment of P. anubis stands in stark contrast with the veneration directed toward P. hamadryas, as attested by massive statues at Hermopolis Magna, a temple 6 km east of Tuna el-Gebel (Figure 1e). The reasons for this distinction—the mummification of P. anubis vs. the iconography of P. hamadryas, both in the service of Thoth—are uncertain, but it was widespread during this time period.
Study design and aims
Isotopic mapping (isomapping) is used to project geospatial variation in the isotope ratios of a given element, together with isotope ratios in biological tissues such as hair, bones, and teeth. The method is useful for estimating the geographic origin and subsequent movement of mobile organisms. Here, our goal is to use oxygen and strontium isomapping to estimate the source of baboons in ancient Egypt. The physiology of baboons is particularly well suited to this approach, although the above evidence of prolonged captivity poses a practical challenge.
Baboons sweat under conditions of thermal stress, turning to sources of meteoric water (pools, streams, lakes) to replenish their body water and maintain homeothermy. Termed 'obligate drinking', this daily behavior produces a strong, mechanistic link between the oxygen isotope ratios (18O/16O) of precipitation and the baboons themselves (Moritz et al., 2012). Such a relationship is advantageous when using oxygen isotope values (δ18O) to trace the regional movements of animals (Bowen et al., 2005b), including primates (Ehleringer et al., 2008); however, it can be difficult to determine region-of-origin from δ18O values alone, a problem that is only exacerbated with increasing time depth.
Another problem is twofold: first, each mummified baboon in our sample died under captive conditions, and it follows that they were provisioned with Nile-sourced drinking water for some portion of their lives (Touzeau et al., 2013), which is expected to undermine the geospatial value of δ18O values in tissues that turn over quickly; and second, we have a limited sample of mixed tissues—hair from EA6736 (Figure 3a); hair, bone, and enamel from EA6738 (Figure 3c); and bone from five skulls, each from the Baboon Catacomb of North Saqqara—representing varying periods of temporal integration. Bone and enamel apatite incorporate years of dietary behavior at different life history stages, whereas hair keratin records information over weeks and months prior to death depending on length.
The enamel of EA6738 holds promise because the mineralization of its permanent incisors occurred early in life, between 1 and 3 years of age (Dirks et al., 2002); and because, in contrast to bone mineral, enamel apatite is resistant to postmortem alteration (Hoppe et al., 2003). It invites measurement of strontium isotope ratios—the 87Sr/86Sr ratio of soils enters foodwebs through leaching by surface waters—together with those of modern baboons for the purpose of isomapping (Bataille et al., 2020). Accordingly, we collected 155 tissue samples from modern baboons representing 77 discrete locations (Appendix 1—tables 1 and 2), and employed a dual-isotope (δ18O; 87Sr/86Sr) approach to estimate the geoprovenance of baboons imported into ancient Egypt.
Results and discussion
Late and Ptolemaic specimens
We measured strontium isotope ratios in the bone apatite of P. anubis (n = 5) recovered from the Baboon Catacomb of North Saqqara and accessioned in the Petrie Museum of Egyptian Archaeology, University College London (Appendix 1—figure 3). The 87Sr/86Sr ratios were strikingly invariant (x̄ =
; Appendix 1—table 3) and indistinguishable from those of ancient Egyptians and Theban carbonate rocks (Figure 4). This finding could result from Sr exchange (diagenesis) with the catacomb itself—indeed, the present samples were probably sourced from piles of macerated, jettisoned bones, the likely result of fourth-century Roman vandalism (Appendix 1—figure 3). Diagenesis from contact with the catacomb walls or dust is therefore plausible (Hoppe et al., 2003), but it fails to account for the distinctly non-local 87Sr/86Sr ratio of a vervet in the same assemblage (Appendix 1—tables 3).
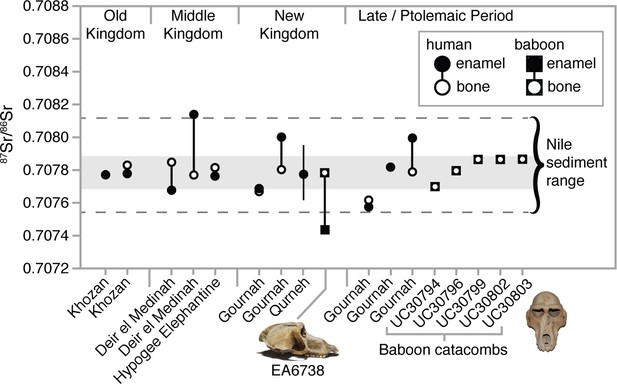
Strontium isotope ratios (87Sr/86Sr) of enamel and bone from humans (Touzeau et al., 2013) and baboons—EA6738 (Papio hamadryas; Thebes) and UC30794-UC30803 (P. anubis; Baboon Catacomb, North Saqqara)—recovered from Egyptian sites.
The data set from Qurneh represents 15 people (mean ± 1 SD; source: Buzon and Simonetti, 2013). The gray-shaded region represents the range of Theban carbonate rocks, whereas the dashed lines define …
In general, the aridity of Egyptian tombs acts to preserve the in vivo (biogenic) Sr isotope values of bone apatite (Touzeau et al., 2013). If the present Sr values from P. anubis are biogenic then they indicate a uniform diet of provisioned food and water that was essentially identical to that of Egyptians living in the Nile Valley. The significance of this interpretation is twofold: first, it corroborates written and osteopathological evidence of prolonged captivity in Egypt, and, by extension, the possibility of a baboon breeding program in Memphis (Ray, 2011); and second, it thwarts our goal of determining the geoprovenance of this sample. Fortunately, our analysis of two New Kingdom specimens proved more revealing.
New Kingdom specimens
We sampled three tissues—enamel, bone, and hair—from EA6738; and, significantly, we detected contrasting 87Sr/86Sr ratios between the enamel (0.707431), a tissue that formed early in life (before 3 years of age), and bone (0.707768), a tissue that is either entirely diagenetic or reflective of the final 5–10 years of life. The former ratio lies beyond the range of Nile sediments, whereas the latter is indistinguishable from those of New Kingdom Egyptians (Figure 4). This result is important for two reasons: it demonstrates that EA6738 was (i) born outside of Egypt and (ii) imported into Egypt where it lived for many years. Thus, we infer that the hair of EA6738—with a mean δ18O value of 16.4 ± 1.2 ‰ (n = 3 replicates)—is a reflection of the water that it drank under captive conditions.
Crucially, the hair of another specimen (EA6736) is substantially more enriched in 18O, with a mean δ18O value of 19.2 ± 0.4 ‰ (n = 5 replicates). The magnitude of this difference is telling, for it indicates the likely retention of geoprovenance-reflecting δ18O values; that is, it would appear that the death and mummification of EA6736 occurred soon (days-to-months) after its arrival in Egypt. Thus, our dual-isotope (δ18O; 87Sr/86Sr) approach to isomapping was based on the hair of EA6736 and enamel of EA6738, respectively.
To estimate the geoprovenance of these tissues, we derived spatially distributed estimates of δ18O and 87Sr/86Sr from the tissues of modern baboons (Appendix 1—tables 1 and 2), and calculated normalized differences against each target tissue, the hair of EA6736 (Figure 5a) and enamel of EA6738 (Figure 5b). It is evident that variation in δ18O does little to constrain our results geographically, but it does refine our sparse sample of bone- and enamel-derived 87Sr/86Sr ratios. Accordingly, we combined the normalized differences of both isotope ratios to visualize an area within 1 SE of both target tissues (Figure 5c). The area encompasses much of present-day Ethiopia, Eritrea, and Djibouti, and portions of Somalia and Yemen.
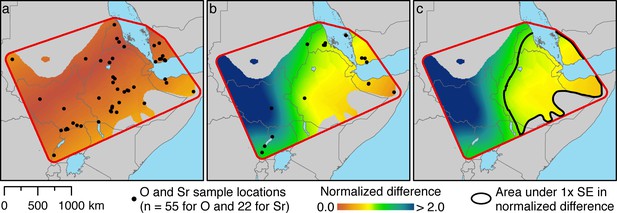
Spatial estimation of isotope ratios and their differences from target values using Empirical Bayesian Kriging.
(a) Specimen locations of modern baboons (black points; source: Appendix 1—table 1) and the normalized difference values for δ18O against our target tissue, the hair of EA6736. (b) Specimen …
A limitation of this analysis is our use of a baboon-derived isoscape, which excludes areas now devoid of baboons, such as Nubia in northern Sudan and southern Egypt. During antiquity, it is plausible that this region accommodated wild or captive populations of export-ready baboons. To explore whether EA6738 could have origins in ancient Nubia, we produced a spatial model (Appendix 1—figure 4) using the global bioavailable strontium isoscape of Bataille et al., 2020. This exercise rules out ancient Nubia as a source of EA6738, but see Box 3 for further discussion.
Box 3.
Considering a Nubian origin for EA6738.
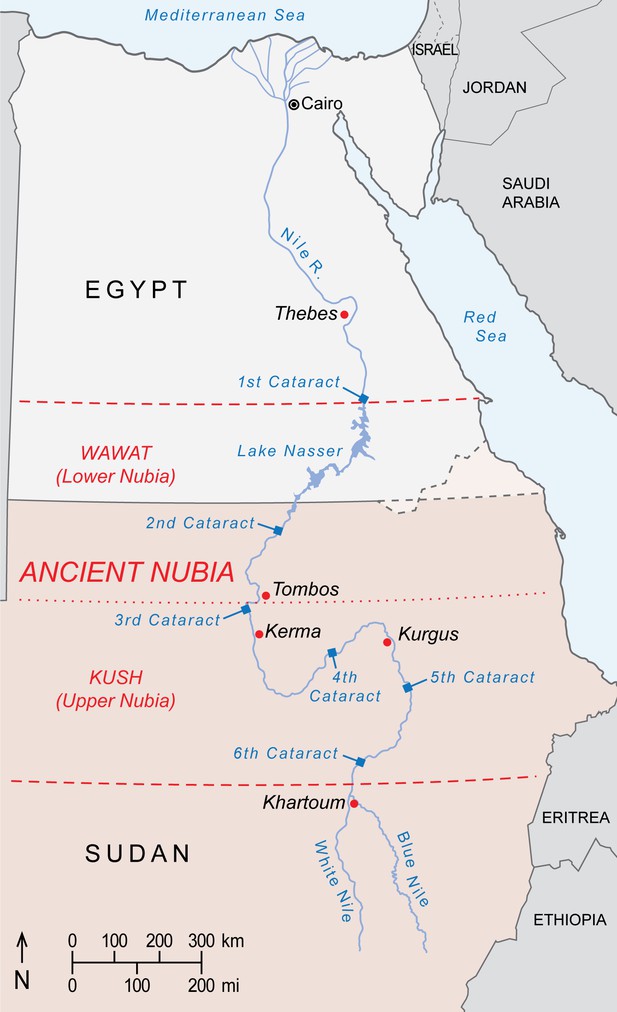
Ancient Nubia is divided into Lower Nubia (including the area between the First and Second Nile Cataracts, referred to as Wawat) and the Kingdom of Kush based at Kerma, Upper Nubia. Much of Nubia may have accommodated baboons during the African Humid Period of the early Holocene, before the shift to hyper-arid conditions around 4500 years ago. Scholars have long presumed that relicts of this former distribution survived into antiquity, and that Nubians captured or raised local baboons for export. But supporting evidence is equivocal, including inscrutable rock art near the Fourth Nile Cataract (Paner and Borcowski, 2005) and accounts of Nubian tribute in the tomb of Rekhmire (Figure 1d) and Papyrus Koller (Gardiner, 1911), neither of which rule out Nubia as an entrepôt for trade goods sourced elsewhere. Yet, the enamel of EA6738 has an 87Sr/86Sr ratio that lies comfortably in the range of values reported for floodplain sediments and human and animal tissues from ancient Nubia (Buzon et al., 2007; Buzon and Simonetti, 2013; Woodward et al., 2015), raising the possibility of a Nubian origin for EA6738. This premise is weakened, however, by Egyptian occupations of Lower Nubia during the Middle Kingdom and again during the New Kingdom, when much of Kush fell to Thutmose I during a military campaign that struck deep into Upper Nubia, reaching Kurgus (Davies, 2005). Had P. hamadryas existed in Wawat or Kush during these periods of Egyptian control, it would have been readily available for export. Yet, there are no physical specimens of P. hamadryas in Egypt prior to the reign of Queen Hatshepsut (Figure 2), the first New Kingdom monarch to resume maritime trade with Punt (Creasman, 2014). We are mindful of the absence-of-evidence fallacy of logic, but it is telling that imports of P. hamadryas from Punt are featured so prominently on the walls of Queen Hatshepsut's mortuary temple (Figure 1c), for it suggests a significant achievement that postdates the conquests of Wawat and Kush. On balance, there is little reason to impute a Nubian origin for EA6738.
Geoprovenance and Punt
A strength of our result is that it puts EA6738 squarely within the natural distribution of P. hamadryas (Figure 2). The significance is twofold: first, it bears witness to the astounding reach of Egyptian seafaring during the 2nd millennium BC; and second, it corroborates reports of long-distance trade with Punt, a toponym of enduring fascination and debate.
Punt occupied a region south and east of Egypt, and was accessible by land or sea. For Egyptians, Punt was a source of 'marvels', particularly aromatic resins, that drove bidirectional trade for 13 centuries (ca. 2500–1170 BC; Figure 2). Some scholars view this commercial enterprise as the beginning of globalization (Fattovich, 2012), whereas others describe it as the beginning of the spice route (Keay, 2006), a trade network that would shape geopolitical fortunes for millennia. The global historical importance of Punt is therefore considerable, but there is a problem, which Phillips, 1997 put succinctly: "Punt has not yet been located with certainty on any map and no archaeological remains have ever been identified even tentatively as Puntite".
Egyptologists of the 19th century were enthralled with Punt, producing at least 54 arguments for its location (Breyer, 2016), but it was the work of Mariette, 1875 that ushered consensus. He put Punt on the Somali coast, an area that produces premium resin from Boswellia frereana (frankincense). A legacy of this era is the modern autonomous state of Puntland in northeast Somalia. Herzog, 1968 broke with this view when he argued for a location in southern Sudan and northern Ethiopia, between Atbara and the confluence of the White and Blue Niles. Kenneth Kitchen then extended Punt eastward to the Red Sea coast of present-day Eritrea (Kitchen, 1971; Kitchen, 1993; Kitchen, 2004), a stretch of Africa favored by most scholars since (O'Connor, 1982; Sleeswyk, 1983; Fattovich, 1993; Bradbury, 1996; Balanda, 2005; Kalb, 2009; Breyer, 2016; Bard and Fattovich, 2018). Some authors have argued for locations as distant as Lake Albert, Uganda (Wicker, 1998) or Mozambique (Lacroix, 1998), but these claims have met with strong criticism or refutation (Phillips, 1999; Bard and Fattovich, 2018). Lastly, sound reasoning exists for the Arabian Peninsula, with Punt representing the whole eastern Red Sea coast as far as present-day Yemen (Meeks, 2003; Tallet, 2013).
Nonhuman primates are germane to this debate because Punt was a major emporium for monkeys. The pyramid causeway of Sahure (ca. 2480 BC) depicts the earliest known expedition to Punt, and monkeys are among the imported goods (El Awady, 2009). Literature provides another example. In The Tale of the Shipwrecked Sailor, a story dated to the Middle Kingdom, an Egyptian sailor is washed ashore on a magical island in the Red Sea. There he meets a serpent identified as the 'Lord of Punt'. When the sailor is rescued, the serpent presents him with many gifts, including long-tailed monkeys and baboons (Phillips, 1997). Speculation that shipwrecked Egyptian sailors were responsible for introducing P. hamadryas to the Arabian Peninsula is a testament to the curious distribution of this species (Kummer, 1981), but the idea is now refuted (Wildman et al., 2004; Winney et al., 2004; Fernandes, 2009; Kopp et al., 2014).
New Kingdom expeditions to Punt imported living specimens of P. hamadryas, as depicted on the reliefs of Deir el-Bahri (Figure 1c) and the tomb-chapels of high officials, dating from Tuthmose III to Amenhotep III. Thus, if the New Kingdom attributions of EA6736 and EA6738 are correct (and the specimens are contemporaneous with voyages to Punt) then our findings corroborate the balance of evidence that puts Punt in (i) the Horn of Africa and (ii) a broader realm, 'God's Land' (Bradbury, 1996), that may encompass the eastern and western coasts of the southern Red Sea (Balanda, 2005; Cooper, 2011). This possibility is attested by Egyptian texts that mention sacred baboons in another Red Sea region called Wetenet, an enigmatic toponym frequently mentioned as the origin of the solar birth and sunrise, evidently in the environs of Punt (Cooper, 2017). It would seem plausible or even probable that Egyptians distinguished between African and Arabian populations of P. hamadryas, but resolving this question is a priority for future research.
Greco-Roman traffic in baboons
Our effort to determine the geoprovenance of five Ptolemaic baboons failed to bear fruit, but thousands of additional specimens exist in the subterranean galleries of North Saqqara and Tuna el-Gebel. It is likely that many of these animals were bred in captivity, but it is equally likely that some were imported via the Red Sea, which raises the possibility of using Sr isotope analysis to establish primatological continuity between Punt and the rise of Axum as the principal supplier of African goods to Roman Egypt (Phillips, 1997). Agatharchides of Cnidus (∼145 BC) described the shipment of baboons from Ethiopia-Eritrea to Alexandria (Burstein, 1989), as well as cepi (probably patas monkeys, Erythrocebus patas [Burstein, 1989]) and sphinx monkeys (probably geladas, Theropithecus gelada [Jolly and Ucko, 1969]). Pliny the Elder named the source port—Adulis (near present-day Massawa, Eritrea)—in his Naturalis Historia, whereas the Egyptian port of Berenice—then a central hub in the maritime spice route, connecting East and South Asia with Egypt and the Mediterranean Basin (Sidebotham, 2011)—is linked to the transshipment of baboons ('cenocephali') on the Tabula Peutingeriana (Geissen, 1984).
Berenice lies ∼250 km south of Mersa Gawasis, a Middle Kingdom harbour and port for seafaring to Punt (Figure 2). It has a rich archaeological record, including ceramics from the Gash lowlands, Eritrea, and Yemen, as well as botanical remains from the coastal plains (and immediate hinterland) of Eritrea, from Aqiq to Adulis (Bard and Fattovich, 2018). It is tempting, then, to suggest that Greco-Roman traffic in baboons between Adulis and Berenice merely followed in the wake of Egyptian sailors navigating between Punt and Mersa Gawasis some 2000 years earlier.
Conclusion
Our effort to map the fabled land of Punt should be viewed as provisional. It is evident, however, that ancient Egyptians venerated P. hamadryas and traveled great distances to acquire living exemplars. Yet, the distribution of baboons is often overlooked when scholars discuss the location of Punt or the luxury goods that drove the evolution of international maritime commerce. Our results suggest that P. hamadryas was an important, contributing factor to the rise of Red Sea trade during the 2nd millennium BC.
Materials and methods
Appendix 1
References
Decision letter
Author response
Essential revisions:
1) The isotopic maps are limited to the modern range of hamadryas baboons. It would be interesting to see a map that includes 87Sr/86Sr values from a broader geographic region including areas north into Sudan and Egypt. While this is not the modern range for hamadryas baboons, is it not possible that their range varied over time? Similarly, wouldn't it have been useful to include areas where hamadryas baboons may have been kept captive and/or bred by humans? We appreciate that samples may be difficult to come by, but we would like to at least see the authors explicitly discuss the potential implications of this limitation on their results.
Thank you for this suggestion, which arrived on the heels of a new and highly relevant publication: Bataille et al., 2020.
Bataille et al. used a random forest regression model that integrates published strontium isotope data and predicts global variation in bioavailable strontium. They caution against extrapolation in "exceptionally geologically complex and data-poor regions", which is the certainly the case for Nubia (see Figure 2 of their paper), but we did so in response to this comment, producing a new figure (Appendix 1—figure 4) and discussionary text:
"A limitation of this analysis is our use of a baboon-derived isoscape, which excludes areas now devoid of baboons, such as Nubia in northern Sudan and southern Egypt. […] This exercise rules out ancient Nubia as a source of EA6738, but see Box 3 for further discussion."
Box 3, in turn, has been revised significantly to address the plausibility of Nubian origins, folding in many of the comments and citations below.
2) In relation to the previous point, one of the reviewers has seen photographs of hamadryas at Erkowit, not far from the Red Sea coast near Suakin, so some of the maps (Box 1 in particular) might show a more northerly extension of these species (or some sort of overlap with olive baboons?). The Erkowit population would be the closest P. hamadryas to Egypt. Would this population have had a similar isotopic signature to others? (We also assume that this population was more widespread in the ancient past). It's also possible the Erkowit baboons have been hunted to extinction in recent history.
Thank you. We are in agreement with the plausibility of this comment, and we have corrected our maps in Figure 2 and Box 1 after verifying the distribution of P. hamadryas on the Sudanese coastline, per Zinner et al., 2013.
3) Some scholars have entertained the existence of baboons in ancient Lower Nubia (especially the prehistoric) and neighbouring regions of the Sudan where they are now no longer found (see Roy, Politic of Trade in Lower Nubia, 266ff). See also the tomb of Djehutihetep at Debeira and Amunedjeh, depicting a baboon in a local Nubian landscape (Kush 8, p. 40; JEA 28, p. 50-52).
Thank you for alerting us to the papers by Davies, 1942, and Säve-Söderberg, 1960. In Davies' view, the paintings in the tomb of Amunedje are "freely imitated from the tomb of Rekhmire, though by an inferior artist". We agree with this assessment and we give the tomb of Rekhmire (Figure 1D and Box 3) precedence for supporting Nubian ties to P. hamadryas, though it should be remembered that trade-goods coming from Nubia did not necessarily originate in Nubia. Nubia itself served as an entrepot for goods from other parts of Africa. The tomb of Djehutihetep at Debeira is fascinating, but Säve-Söderberg's sketch is decidedly ambiguous. To our eye, and based on the length of the tail, the primate is a vervet / green monkey in the genus Chlorocebus. Indeed, Säve-Söderberg himself seems conflicted, describing the animal alternately as a "monkey" or "baboon" before stressing disparities between it and typical depictions of baboon-fruit interactions. In our view, neither paper rises to the level of corroborating a Nubian origin for P. hamadryas during the 18th Dynasty.
So too, the present day Eastern and Western Desert populations of baboons in Sudan (Gebel Mara, Erkowit, Khor Langeb) seem to be relicts of wider distributions of baboons in wetter periods. Papyrus Koller also seems to demonstrate baboon imports from Nubia, specifically Kush (Gardiner, Late Egyptian Miscellanies, 112). There are some indications of baboons in the rock art of Gilf Kebir and also the Fourth Cataract in Nubia (Kleinitz, Rock art at the Fourth Nile Cataract: An overview).
Thank you for alerting us to the work of Gardiner, 1937, which led us to his earlier work translating the Papyrus Koller, published in 1911. The collection of papyri includes a letter from a high official named Paser to a Nubian chieftain, ordering him to make ready a long list of tribute objects, including baboons (Gardiner, 1911). This point and citation are now folded into Box 3 of our manuscript. But it should be remembered that trade-goods from Nubia did not necessarily originate there. Nubia often served as an entrepot for goods from other parts of Africa.
We consulted the 2012 paper by Kleinitz, but on the subject of baboons it cites the work of Paner and Borcowski, 2005. When asked if she has encountered images of baboons, Dr Kleinitz replied, "I have not seen any during my study of rock art at the Fourth Cataract. However, they are part of the Meroitic canon of motifs and we find quite a lot of them as graffiti on temple walls.". Such images are too recent to support the premise of relict baboons inhabiting Kush or Wawat, and indeed, could depict animals brought in through trade. In the paper by Paner and Borcowski, 2005, an image that resembles a baboon is reproduced on page 115. The tail, however, is curved upwards rather than downwards, and the length is excessively long relative to body size. It is more likely a dog, not a baboon, as the ears also suggest a dog rather than a baboon, falling well within the canon of canine images in rock art of Sudan/Nubia and Egypt. If the original artist intended to illustrate a baboon, it is an equivocal likeness that cannot be attributed to either Papio anubis or P. hamadryas. Paner and Borcowski, 2005, also stress the challenge of dating rock art in the region. Taken together, we could not find compelling evidence from rock art to support the idea of natural populations of baboons in ancient Kush or Wawat. Still, the possibility is now expressed, and the paper by Paner and Borcowski is now cited, in Box 3 of our manuscript.
In a more general tenor on the possibility of Sudanese populations, there is overwhelming archaeological and palaeoclimatic data for changing ecologies in Sudan, some of which was ongoing throughout the pharaonic period. Cattle, for instance, were still found in the Eastern and Western deserts of Sudan in the relevant periods, which argues for a much wetter climate and possibilities of ecologies that would have sustained baboons.
We have edited Box 3 to describe the African Humid Period of the early Holocene, and the shift to hyper-arid conditions around 4500 years ago, as well as the possibility of baboons occupying northern Sudan and surviving into antiquity. A problem with expressing this view is that Papio anubis was the probable occupying species, not P. hamadryas, but such a statement puts us in the awkward position of responding to speculation with further speculation. In any case, Box 3 was written to acknowledge the possibility of baboons in ancient Nubia and simultaneously weigh the evidence for a Nubian origin of EA6738. In addition, it is clear that the climate was variable, as even in New Kingdom Egypt Farafra Oasis, in Egypt's Western Desert, was known for supporting cattle herds. There is evidence for small areas with microclimates to support cattle, but neither pictorial or textual evidence support the existence of baboons in the area.
On this point, one might add that there was another important Red Sea placename in Egyptian texts (religious, medical, and expedition texts) from which baboons derived, called 'Wetenet' in Egyptian texts. The placename is consistently linked with baboons in religious texts -- but is also a real place reached on Egyptian expeditions (see Muller-Roth, Das Buch vom Tage, 155; Cooper 'Between this world and the Duat: The Land of Wetenet and Egyptian Cosmography of the Red Sea', in. C. Di Biase-Dyson and L. Donovan (eds), The Cultural Manifestations of Religious Experience: Studies in honour of Boyo G. Ockinga, 383-394; Edel, Beiträge zu den ägyptischen Sinaiinschriften). This might also have consequences for locating hamadryas/olive baboon populations, as some of these texts provide defining features of the baboons (dark skin, red-eyes). Adding all this discussion may be admittedly beyond the remit of the paper, but we think it is worth mentioning the placename at least with respect to the historical context/framework in the Introduction.
Thank you for alerting us to Wetenet and its association with baboons and Punt. We have happily folded this topic into our Discussion:
"Thus, if the New Kingdom attributions of EA6736 and EA6738 are correct (and the specimens are contemporaneous with voyages to Punt), then our findings corroborate the balance of evidence that puts Punt in (i) the Horn of Africa and (ii) a broader realm, "God's Land" (Bradbury, 1996), that may encompass the eastern and western coasts of the southern Red Sea (Balanda, 2005; Cooper, 2011). […] It is plausible or even probable that Egyptians distinguished between African and Arabian populations of P. hamadryas, but resolving this question is a priority for future research."
https://doi.org/10.7554/eLife.60860.sa2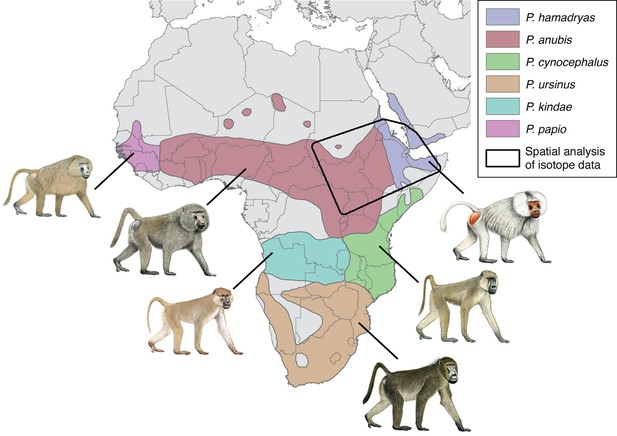

No comments:
Post a Comment